Background and overview[1]
2,3-Difluorophenylacetic acid is a widely used pharmaceutical intermediate and an important raw material for the preparation of new liquid crystals. It has huge market demand and broad prospects.
Preparation[1]
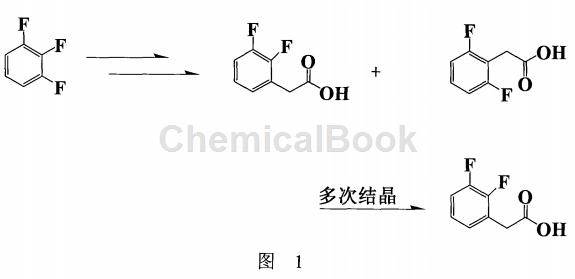
In the synthesis strategy of 2,3-difluorophenylacetic acid, due to the particularity of its structure, for example, it is planned to attach a group to the carbon next to the fluorine atom and use the traditional Fulcker reaction method to obtain the product. There are many positional isomers, which is not only difficult to separate, but also the target product is not the main product. If a general nucleophilic substitution reaction is used, such as using 1,2,3-trifluorobenzene as the reaction subject (Figure 1), the raw materials are expensive. In addition, the isomers produced by side reactions are difficult to remove (it must be crystallized multiple times to obtain a pure product), resulting in a low yield (<30%), which does not meet the requirements of industrialization (B.A. Kowalczyk, Synthesis, 1411 (1997) ).
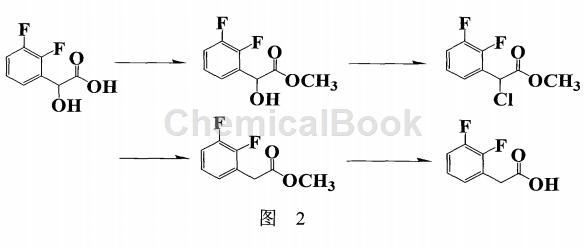
German patent WO2008078350 (Figure 2) reports that 2,3-difluoro-mandelic aid is used as a starting material to prepare 2,3-difluorophenylacetic acid after esterification, chlorination, reduction, alkaline hydrolysis and acidification. Due to the numerous steps, the yield is low (44.2%), and the starting materials are expensive and are not suitable for industrial production.
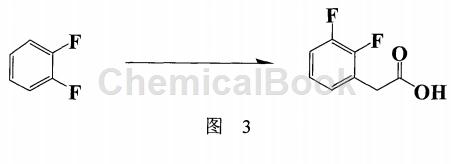
In addition, Japanese patent: JP2004352724 (also applied for Chinese patent: CN100378062C) (Figure 3) proposes to use o-difluorobenzene as the starting material, and n-butyllithium and ethyl 2-bromoacetate in the presence of two catalysts The reaction produces ethyl 2,3-difluorophenyl acetate with a maximum yield of 77%. After saponification reaction and recrystallization are carried out, the total yield of the product is reduced to 51.92%. Furthermore, the catalyst used is The reaction result has a great influence (total yield 8% ~ 77%), but this catalyst [bis(acetylacetonate-2,2′-bipyridyl nickel (II)] is not easy to buy due to its special structure, and it is not known whether it can be repeated Apply to reduce costs. Preparing such effective catalysts yourself may be expensive, so it is not the best way to industrialize it.
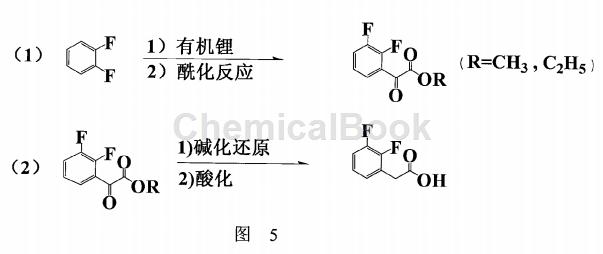
CN200910030821.9 provides a preparation method with simple process flow, mild reaction conditions, easy post-treatment cleaning, high product purity, low cost and suitable for industrial production. The specific steps are:
(1) At low temperature, o-difluorobenzene reacts with organic lithium to generate o-difluorophenyllithium, which then reacts with oxalate ester or chlorooxalate to generate 2,3-difluorophenyllithium. Fluoroacetophenoate;
(2) The 2,3-difluorophenylacetic acid ester is reduced under alkaline conditions, and then acidified and precipitated to obtain 2,3-difluorophenylacetic acid, which is then crystallized and purified. As shown in Figure 5:
The specific operations are as follows:
Preparation of ethyl 1,2,3-difluoroacetophenonate
Add 50g (0.438mol) o-difluorobenzene and 400ml anhydrous THF into a 2L three-neck bottle (A), then seal it and fill it with N2, and then cool it to -70℃ And start to drop 176ml (0.440mol) of 2.5M n-butyllithium (2 hours in total). After the dripping is completed, keep stirring at -65~-75°C for 2 hours; at the same time, add another 2L three-necked flask (B ), place 67.2g (0.46mol) of diethyl oxalate and 100ml of anhydrous THF, then seal it and fill it with N2 and then cool it to -70°C. Use N2 to slowly press the material from bottle (A) into bottle (B) (it takes 1 hour and controls the temperature within the range of -65 ~ -70 ℃), and then continue within this temperature range. React for 1 hour and naturally bring the temperature to 0°C (1 hour), and then add 60 ml of 6N HCl dropwise to the aforementioned reaction mixture (30 minutes, open the temperature to 15°C, pH 3~4). After adding HCl dropwise, add this The mixture was stirred for 30 minutes and allowed to stand for layering. The lower emulsion aqueous layer was extracted twice with 80 ml of ethyl acetate. The organic layers were combined and directly desolvated and purified by distillation to obtain ethyl 2,3-difluoroacetophenonate. (Light yellow liquid): 85.1g, content: 96% (HPLC), yield: 90.8%.
Preparation of 2, 2,3-difluorophenylacetic acid
Put 91g (0.425mol) of 2,3-difluoroacetophenonate ethyl ester into a 1L single-neck bottle and add 270ml H2O to dilute, then add dropwise 57.3g ( 0.51mol) of 50% KOH and continue stirring for 1 hour (TLC shows that it has been completely converted into potassium hydride); then add 90ml of n-hexane and stir for 30min to remove impurities in the organic layer, and move the separated aqueous layer to another clean 1L In a three-necked bottle, add 180ml H2O at room temperature (20~25℃) to dilute, then add dropwise 57.3g (0.51mol) of 50% KOH and 39.9g (0.638mol) of 80% hydration hydrazine (30 min each, temperature ~28°C), then continue stirring for 1 hour, then raise the reaction temperature to 85~95°C within 30 minutes and continue the reflux reaction for 16 hours.
Then the temperature of the reaction system was lowered to room temperature (1 hour), 230 ml of 6NHCL was added dropwise and the pH was adjusted to 1 (30 minutes, temperature ~30°C) and stirring was continued for 30 minutes. At the same time, a large amount of white solid precipitated, which was filtered And wash it with 100ml H2O 2 to 3 times until the pH of the filtered water is 3 to 4. Then pump the white solid and place it in an oven (~85°C) for 4 hours to obtain 69.5g. 2,3-Difluorophenylacetic acid crude product, content (HPLC)>96%. The aforementioned crude product was crystallized with methanol and H2O = 1:3 to obtain 62.53g of pure off-white 2,3-difluorophenylacetic acid, content (HPLC): 99.6%, yield 85.5%.
Main reference materials
[1] CN200910030821.9 Industrial preparation method of 2,3-difluorophenylacetic acid

 微信扫一扫打赏
微信扫一扫打赏

