Preparation method【1】【2】【3】【6】
1. In a 100ml three-necked flask, add 10.0g of 2,6 difluorobenzonitrile, 30ml of H2O2, 40ml of alcohol and some homemade catalysts, control the temperature to 40~50℃, add 30ml of OH- dropwise, after adding Incubate the reaction for 4 hours. After the reaction, steam distillation (part of the distilled alcohol is recovered and used), cooling and crystallization, filtering, and drying to obtain 9.7g of 2,6 difluorobenzamide, purity 99%, mp 145~147°C, yield 97%.
Add 65g of prepared alkali into a 100ml three-necked flask, cool to below 0℃, add 10.2g of bromine and 9.7g of 2,6 difluorobenzamide, react below 0℃ for 1 hour, release and move to 200ml In a three-necked flask, add 40g of NaOH and a certain amount of NaHSO3 in sequence, conduct the Hoffmann reaction for 30 minutes, and control the temperature at 60 to 70°C. After the reaction is completed, use steam distillation to separate the organic layer, and collect the 50~60°C/1.33kPa fraction through vacuum distillation to obtain 6.8g of 2,6 difluoroaniline with a purity of more than 99% and a yield of 70%.
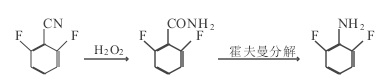
Figure 1 shows the reaction route 1 for the synthesis of 2,6-difluoroaniline
2. Use 2,6-difluorobenzamide, sodium hypochlorite, etc. as raw materials to synthesize 2,6-difluoroaniline.
(1) Bromine method
Add the prepared NaOH solution to a 25OmL three-neck flask, cool to below 0°C, add 11.3g of bromine in portions, stir for 15min, 10.6g of 2,6-difluorobenzamide, 0°C The following reaction is 1 hour, add a certain amount of NaOH, react for 0.5 hours, control the temperature at 60-70°C, add a certain amount of NaHSO4, and stir for 10 minutes. After the reaction is completed, steam distillation is performed to separate the organic layer, and the 50-60°C/l.33kPa fraction is collected through vacuum distillation to obtain 7.5g of 2,6-difluoroaniline with a purity of over 99% and a yield of 70%.
(2) Chlorine gas method
In a 250mL three-neck flask, add 140g of water, some NaOH, 40g of benzyl alcohol and 25.8g of 2,6-difluorobenzamide. Pass Cl2 at a speed of 5L/h, and control the temperature at 20-30°C. After 1 hour of reaction, stop flowing Cl2, add some NaHSO4, stir for 10 minutes, and the reaction is completed. Distill with water vapor, separate the organic layer, and then distill under reduced pressure to obtain 20.7g of 2,6-difluoroaniline. The product can be purified by distillation, and the purity can reach more than 99%. The yield is over 80%.
(3) Sodium hypochlorite aqueous solution method
In a 25OmL three-necked flask, add 80g NaOH and 205mL NaClO aqueous solution respectively, cool to below 0°C, add 17.2g 2,6-difluorobenzamide in portions, and stir for 1 hour. Add NaSHO4 and stir for 10 minutes. After the reaction is completed, distill with water vapor to separate the organic layer and then distill under reduced pressure to obtain 13.8g of 2,6-difluoroaniline with a purity of more than 99% and a yield of more than 80%.
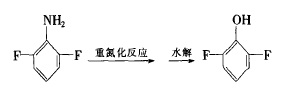
Figure 2 shows the reaction route 2 for the synthesis of 2,6-difluoroaniline
3. The synthesis of 2,6-difluoroaniline by the 2,6-dichlorobenzonitrile method mainly undergoes the three-step reaction of fluorination, hydrolysis and Hofmann reaction, as shown in Figure 3.
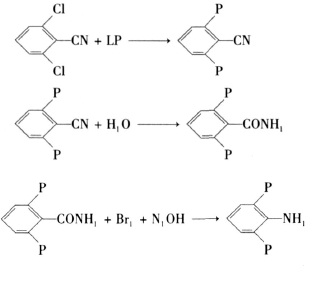
Figure 3 shows the reaction route 3 for the synthesis of 2,6-difluoroaniline
Fluorination reaction steps: Add a measured amount of 2,6-dichlorobenzonitrile, potassium fluoride, catalyst and solvent into a four-neck reaction bottle equipped with a stirrer, heat the reaction, and vacuum distill after the reaction to obtain 2 , 6-difluorobenzonitrile products.
Hydrolysis reaction steps: In a vessel reaction bottle equipped with a stirrer, add the prepared sulfuric acid and DFBN in measured amounts. The reaction temperature is 60 to 80°C, and the reaction is for 4 to 6 hours. After the reaction is completed, the reaction solution is poured into ice water, the product crystals are precipitated, filtered with suction, washed with a small amount of ice water, and dried to obtain the 2,6-difluorobenzamide product.
Hoffmann rearrangement reaction steps: In a four-neck reaction flask equipped with a stirrer, thermometer, and condenser, add the prepared base and 2,6-difluorobenzamide in an ice-water bath, and drop in bromine The reaction is carried out with bromine. After the bromine is dropped, the temperature is raised to 60-70°C and the reaction is carried out for 2 hours. The reaction liquid is still separated into layers to obtain crude 2,6-difluoroaniline, which is then distilled to obtain the final product.
4. Use 2.6-dichlorobenzonitrile as raw material to synthesize 2.6-difluoroaniline through fluorination, hydrolysis and Hofmann reaction, with a yield of 73.4%.
(1) Synthesis of 2.6-difluorobenzonitrile,
In a four-neck reaction flask equipped with stirring and thermometer, add 17.37g (0.1mol) 2.6-dichlorobenzonitrile, 15.26g (0.25mol) anhydrous potassium fluoride, 150ml sulfolane and catalyst and heat to 170 – React at 180°C for 2 hours, then raise the temperature to 230-240°C and react for 4 hours. The reaction product is vacuum distilled to obtain 12.50g of 2.6-difluorobenzonitrile, with a yield of 88.1% and a content of ≥ 98%.
2. 2.6—Synthesis of difluorobenzamide.
Add 2.6-difluorobenzonitrile 14.20 (0.1mol) and 60g of 90% sulfuric acid into a four-necked reaction flask equipped with a stirrer, thermometer, and condenser tube, and raise the temperature to 70-80°C for 4 hours to react. Add water to precipitate white crystals. After washing and drying, 15.02g of 2.6-difluorobenzamide is obtained, with a content of 99% and a yield of 94.7%.
3. Synthesis of 2.6-difluoroaniline
Add 2.6-difluorobenzamide 15.86 (0.1mol), prepared base 100g (0.125mol), and cosolvent into a four-neck reaction bottle equipped with a stirrer, thermometer, condenser tube, and air guide tube and cool to 0-5℃, pass in chlorine, react for about 2 hours until the solution is clear, then stop. The reaction solution is steam distilled, and the organic layer is separated to obtain crude 2.6-difluoroaniline. After further distillation, 11.58g of the target substance is obtained, with a content of 98 %, yield 88%.
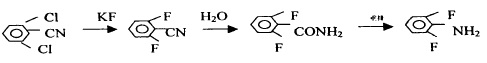
Figure 4 shows the reaction route 4 for the synthesis of 2,6-difluoroaniline
Application【1】【4】
1.2,6 difluoroaniline is a synthetic herbicide��Intermediates of flumetsutam.
2.2,6-Difluoroaniline is an important intermediate for the preparation of benzamide herbicides, insecticides and liquid crystal materials. Herbicides prepared using 2,6-difluoroaniline as intermediate raw materials, such as the herbicide “Broakstrike” successfully developed by the American Company in the early 1990s, are highly effective in the environment due to their high efficiency, broad spectrum, high selectivity, and low toxicity. It does not accumulate and is highly safe for humans and animals. It is widely used in Western countries and is known as a green and environmentally friendly pesticide.
3.2.6—Difluoroaniline is a fine chemical product with broad application prospects, mainly used in the production of benzoyl urea pesticides and liquid crystal materials.
Main reference materials
[1] Lu Hongchu. Synthesis of 2,6-difluoroaniline [J]. Liaoning Chemical Industry, 1997(05):37.
[2] Zhu Hong, Huang Shan. Synthesis of 2,6-difluoroaniline and 2,6-difluorophenol [J]. Chemical Engineering Times, 1999(12):39-40.
[3]Xiong Guolan, Jiang Baiquan, Lai Xiaobo. Research on the synthesis process of 2,6-difluoroaniline, a high-efficiency pesticide raw material [J]. Anhui Agricultural Sciences, 2008(23):9848-9849.
[4] Zhang Yanshun. Synthesis of the herbicide 2,6-difluoroaniline [J]. Guizhou Chemical Industry, 2001(01):31-32.
[5] Zhao Hui, Zhao Longhui, Zhao Bin. Synthesis of 2,6-difluoroaniline [J]. Hebei Chemical Industry, 2003(03):29-30.
[6]Huang Bin, Song Liyan, Yu Wenxue, Fu Guiyun, Yi Jin. Synthesis of 2.6-difluoroaniline [J]. Jiangxi Chemical Industry, 2006(01):83-84.

 微信扫一扫打赏
微信扫一扫打赏

