Background and overview[1][2]
Diaminodihydroxybenzene can be used as a monomer for the preparation of polybenzoxazole, by combining 2,5-diamino-1,4-dihydroxybenzene dihydrochloride with diacid, dicarboxylic acid halide, dihydrobenzene Polybenzoxazole can be produced by reacting esters or dinitriles. In order to obtain a high molecular weight polybenzoxazole that can be effectively spun into suitable fibers, a very pure starting material is required. Polybenzoxazole prepared from high-purity 2,5-diamino-1,4-dihydroxybenzene dihydrochloride can be spun into fibers with high tensile strength and good thermal stability. Such fibers are suitable for military, aerospace and applications requiring high-performance materials.
The traditional method of preparing 2,5-diamino-1,4-dihydroxybenzene dihydrochloride involves treating diacetyl-1,4-dihydroxybenzene with white nitric acid. When treated with nitric acid, unwanted 2,2,5-trinitro-1,4-dihydroxybenzene is formed, and repeated recrystallization is required to separate the required 2,5-dihydroxybenzene from the unwanted by-products. Nitro-1,4-dihydroxybenzene. Then, 2,5-dinitro-1,4-dihydroxybenzene is catalytically hydrogenated in dilute hydrochloric acid to obtain 2,5-diamino-1,4-dihydroxybenzene dihydrochloride. The disadvantages of this method are that it requires good purification and the starting materials are expensive.
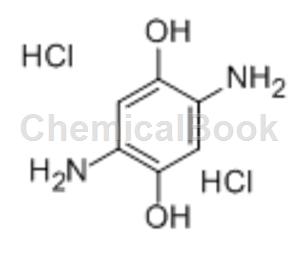
Preparation[2]
1.Dinitration of 1,2,3-trichlorobenzene
Add 544.4 grams of 1,2,3-trichlorobenzene and 3,425 grams of 96.5% sulfuric acid into a 5-liter three-neck round-bottomed flask equipped with a mechanical stirrer, condenser and addition funnel. . The reactor was placed in a variable temperature bath and the temperature was raised to 65°C. During this period, add 594.1 grams of 71% concentrated nitric acid dropwise at a rate that maintains the reaction temperature at 65°C. After the addition is completed, the reaction mixture is maintained at 65°C until 98 to 99% of the reaction mixture has been converted to dinitrotrichlorobenzene by gas chromatography (usually 1 to 3 hours) (gas chromatography conditions As follows: about 25M DB-5 capillary chromatography column, furnace temperature: 100~250℃, flame ionization detector, programmed temperature rise: 20℃/minute).
After the reaction is completed, add 237.1 grams of water dropwise. The dripping rate should keep the reaction temperature between 45°C and 65°C. Maintain vigorous stirring (≤300 rpm) throughout the reaction. After this time, the reaction mixture was cooled to room temperature, and the product was filtered off, washed with 2.2 liters of water, and air-dried to obtain 773.5 g of essentially pure 1,2,3-trichloro-2,5-dinitrobenzene (isolated yield approx. (95%) (the yield is usually 93 to 98%). It was used without further purification.
2.1,4-Dihydroxy-2-chloro-2,5-dinitrobenzene
Put 200.3 grams of 1,2,3-trichloro-2,5-dinitrobenzene (0.74 mol), 258.0 grams of methanol and 717.1 grams of water into a 4-neck round-bottomed flask with a volume of 5 liters. The reaction mixture was stirred at room temperature and 358.4 g of 50% NaOH solution were added to the mixture in one portion. At this time, a slight exothermic phenomenon can be observed, and the reaction system is heated to 80°C. When the reaction temperature reaches 75°C, the reaction system is homogeneous, and another exothermic reaction can be observed to occur, accompanied by violent reflux. The solution turns dark red and its temperature rises to 85°C.
After this phenomenon, the disodium salt of the product precipitates out of the solution. The temperature was maintained at 75°C to 80°C until complete conversion was observed by liquid chromatography (conditions: 2 cm RP-2 guard column, 15 cm Zorbax phenyl column, and 25 cm WhatmanSCX strong cation exchange column, solvent 30 % acetonitrile aqueous solution is buffered to pH 2.80 with 0.02M KH2PO4 and 85% H3PO4; the flow rate is 2.0 ml/min at room temperature, and the aliquot of the reaction is neutralized with concentrated hydrochloric acid diluted with water and then dissolved in CH3CN) (the conversion rate is usually 99.6% to 99.8%).
After the reaction is completed, the reaction mixture (thick slurry) is cooled to room temperature and neutralized with 331.3 grams of concentrated hydrochloric acid (approximately 35%). Care should be taken to keep the temperature below 40°C when adding HCl. The obtained slurry was then extracted with 1772.4 g of ethyl acetate, and the solvent was concentrated under vacuum. The resulting residue was washed with 104 g of methanol, separated by filtration and air-dried to obtain 157.0 g of 1,4-dihydroxy-2-chloro- 2,5-dinitrobenzene (isolated yield 90.8%), the purity determined by liquid chromatography is 99.886%.
3. Preparation of 2,5-diamino-1,4-dihydroxybenzene dihydrochloride
Put 117.3 grams (0.5 mol) of 1,4-dihydroxy-2- into a 1-liter corrosion-resistant nickel-based alloy (Hastalloy) C-type autoclave equipped with a gas dispersion stirrer and a cooling coil. Chloro-2,5-dinitrobenzene, 400 ml of glacial acetic acid, 41 g (about 0.5 mol) NaOAc, about 7.0 g of 10% Pd/C and 100 ml of water. H2 is introduced into the sealed reactor so that the pressure reaches 400 pounds per square inch and the temperature reaches 40°C. During the reaction, the temperature is maintained at 40°C to 50°C. After a short induction period, the consumption of hydrogen accelerates sharply, and the pressure of H2 must be maintained at 100 to 400 pounds per square inch during the reaction. When the reaction is complete, no consumption of H2 is observed.
After that, cool the reactor to room temperature, open the reactor, and add 400 ml of concentrated hydrochloric acid containing 10 grams of SnCl2·2H2O. Filter and separate the crude product with catalyst, dissolve it in 200 grams of water at 85°C and filter out the catalyst. Add water (100 to 300 ml) and 500 ml of HCl to the above filtrate. Materials without catalyst are removed from the filtrate. Precipitated out of brown solution. Recrystallize in the original solvent, or separate the semi-pure material and air-dry to obtain 100 grams of diamino intermediateDiphenol dihydrochloride is the crude product of 2,5-diamino-1,4-dihydroxybenzene dihydrochloride [product based on 2,5-diamino-1,4-dihydroxybenzene] The rate is 96.8% (molar)].
Main reference materials
[1] Yang Zhou. (2010). 4-(4-Dimethylaminostyryl)methylpyridine 3-carboxy-4-hydroxybenzenesulfonate, its nonlinear optical crystal and preparation method.
[2] He Bingjing, & Tao Xuefen. (2008). Synthesis of 2-amino-5-p-hydroxyphenyl-1,3,4-thiadiazole and its Schiff base. Synthetic Chemistry, 16(2 ), 233-236.

 微信扫一扫打赏
微信扫一扫打赏

